Early in the 20th century, dentists discovered that fluoride reduced the number of cavities in their patients’ teeth. Soon, amidst much debate and rancor, fluoride was regularly added to American public drinking water supplies. Fluoridation has been hailed by some as a triumph of public health but it was also, as historian Frank Zelko points out this month, a profitable way to put to use a waste byproduct from the production of fertilizer.
While Florida calls itself the Sunshine State, from a geological and economic perspective, it could just as accurately be known as the Phosphate State.
The so-called Bone Valley of central Florida contains some of the largest phosphate deposits in the world, which supply global agriculture with one of its most important commodities: synthetic fertilizer. In the process, the mining industry leaves behind a scarred landscape denuded of vegetation and pocked with vividly colored waste disposal ponds that one writer described as “beautiful pools of pollution.”

Phosphate loaded by elevator at Port Tampa, FL in 1958.
Highly toxic hydrogen fluoride and silicon tetrafluoride gases are by-products of fertilizer production. Prior to the 1970s, these pollutants were vented into the atmosphere and gave central Florida some of the most noxious air pollution in the country.
During the 1960s, however, complaints by farmers and ranchers eventually forced reluctant manufacturers to invest in pollution abatement scrubbers that converted toxic vapors into fluorosilicic acid (FSA), a dangerous but more containable liquid waste.
The U.S. National Institute for Occupational Safety and Health (OSHA) cautions that FSA, an inorganic fluoride compound, has dire health consequences for any worker that comes into contact with it. Breathing its fumes causes severe lung damage or death and an accidental splash on bare skin will lead to burning and excruciating pain. Fortunately, it can be contained in high-density cross-linked polyethylene storage tanks.
It is in such tanks that fluorosilicic acid has for the past half century been transported from Florida fertilizer factories to water reservoirs throughout the United States. Once there, it is drip fed into drinking water. This is a practice that the American Dental Association and numerous scientists and public health officials describe as “the precise adjustment of the existing naturally occurring fluoride levels in drinking water to an optimal fluoride level … for the prevention of dental decay.”

A worker watching the loading of powder fine phosphate in Mulberry, FL in 1947 (left). An 1892 map of phosphate deposits on the western edge of Florida (right).
The practice of adding fluoride compounds (mostly FSA and occasionally sodium fluoride) to drinking water is known as community water fluoridation. It has been a mainstay of American public health policy since 1950 and continues to enjoy the support of government health agencies, dentists, and numerous others in the medical and scientific community.
As with many chemical additives in the modern world, however, few people know much about it.
Many are surprised to learn that unlike the pharmaceutical grade fluoride in their toothpaste, the fluoride in their water is an untreated industrial waste product, one that contains trace elements of arsenic and lead. Without the phosphate industry’s effluent, water fluoridation would be prohibitively expensive. And without fluoridation, the phosphate industry would be stuck with an expensive waste disposal problem.
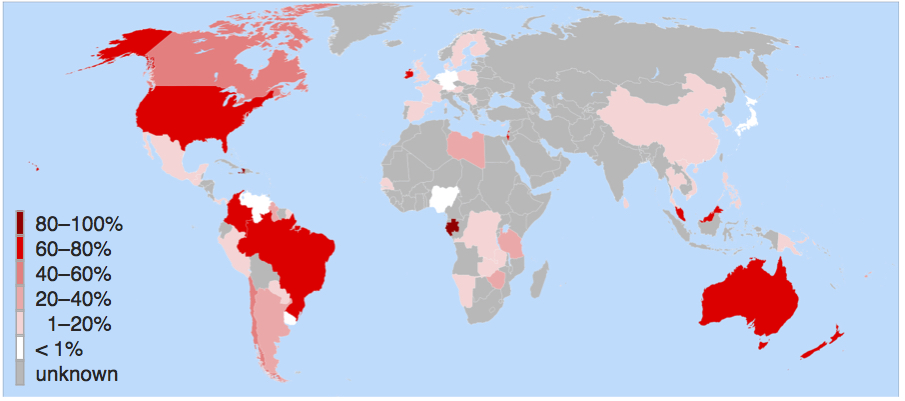
Only a handful of countries fluoridate their water—such as Australia, Ireland, Singapore, and Brazil, in addition to the United States. Western European nations have largely rejected the practice. Nonetheless, dental decay in Western Europe has declined at the same rate as in the United States over the past half century. In fact, the more one looks at the history of fluoridation, the more it appears to be a relic of the sort of mid-20th century scientific incaution that gave us DDT, thalidomide, and other attempts at “better living through chemistry.”
This is not to vilify the early fluoridationists, who had legitimate reason to believe that they had found an easy and affordable way to counter a significant public health problem. However, the arguments and data used to justify fluoridation in the mid-20th century—as well as the fierce commitment to the practice—remain largely unchanged, failing to take into account a shifting environmental context that may well have rendered it unnecessary or worse.

An advertisement for the pesticide DDT from Time magazine in 1947 (left). An advertisement from the 1940s for children's wallpaper laced with DDT (right).
Ugly Smiles and Tough Teeth
Fluoride’s public health history is like a crime story with a twist. After following a trail of clues for many years, detectives finally catch their chief suspect and put him on trial. But it soon turns out that he has redeeming qualities that far outweigh the crime for which he was originally charged.
The indefatigable private eye in this case was a young Massachusetts-born dentist, Frederick McKay. After completing his training at the University of Pennsylvania School of Dentistry, McKay moved to Colorado Springs in 1901 to establish his first practice.

He soon became perplexed by the unsightly tea-colored stains that discolored many of his patients’ teeth, a condition that he was unable to find in the dental literature. McKay began calling it “brown stain” and “Colorado stain,” and nobody understood why many residents of that particular region suffered from it while those in neighboring counties did not. In the summer of 1909, McKay and some colleagues inspected the mouths of 2,945 Colorado Springs children and discovered that 87.5% suffered from the condition.
Upon further investigation, McKay determined that the Colorado Springs area was not unique. There were pockets of brown stain throughout the country. McKay began to conduct an informal epidemiological study. He examined the local diet, soil conditions, and air quality, but eventually decided that the culprit had to be the water.
“The evidence is so conclusive,” he wrote in 1927 to the Public Health Service (PHS) in Washington, D.C., “that it is futile to discuss it further from any other standpoint.” Despite testing numerous samples, however, he could not find anything unusual in the local water supply, which was clear, odorless, and agreeable to the taste. Nevertheless, he became increasingly convinced that some as yet undetected trace element in the water was responsible for the dental lesions.
A big step toward solving the mystery of brown stain occurred in 1931, when nervous chemists at the Aluminum Company of America (ALCOA) began to examine the water in Bauxite, Arkansas. The principle ore of aluminum, bauxite was vital to ALCOA’s production process. In 1909, the town’s growing population necessitated a new water supply, and ALCOA dug three deep wells to access the ample groundwater. In a few years, children in Bauxite began to be afflicted with brown stain. Initially, this was of no great concern to ALCOA. By the late 1920s, however, the company was fending off charges that its aluminum cookware was slowly poisoning the population.

The logo for the Aluminum Company of America (left). A mural of bauxite miners from the 1940s in Benton, AR (right).
ALCOA's chief chemist, H. V. Churchill, was concerned that any link between aluminum and brown stain would be a public relations disaster. So in 1930, he tested Bauxite’s water supply using the most advanced spectrographic equipment available at the time. The tests showed that the groundwater had unusually high levels of the element fluorine—15 parts per million (ppm), a result, he wrote McKay, “so unexpected in water that a new sample was taken with extreme precautions,” but showed the same outcome.
While McKay and Churchill were busy revealing fluoride’s undesirable effect on human teeth, a young Danish scientist, Kaj Roholm, was investigating the impact of industrial fluoride on human health.
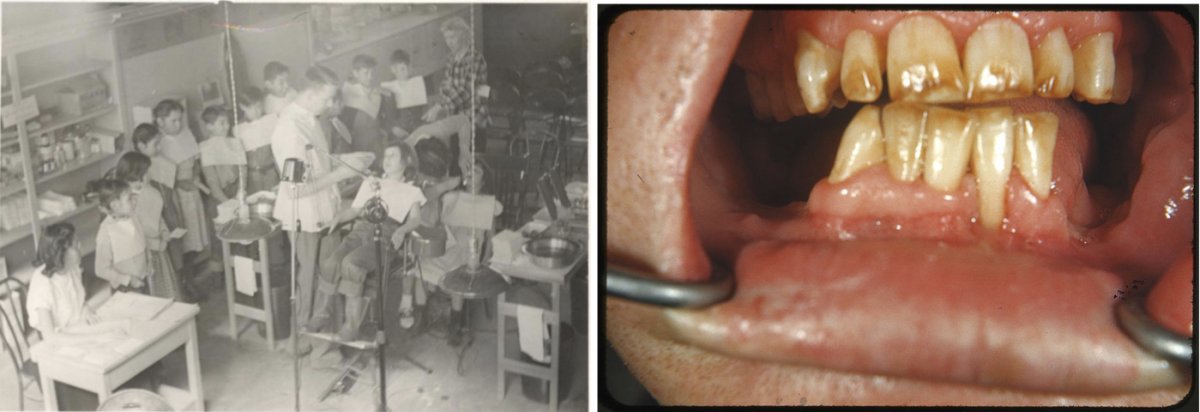
A dentist examining children’s teeth at the Pine Ridge Indian Reservation in the 1940s or 1950s (left). Severe fluorosis, brown discoloration and mottled enamel, in an individual from an area of New Mexico with naturally occurring fluoride in the water (right).
In 1930, a dense layer of polluted fog settled over the Meuse Valley, a heavily industrial area in eastern Belgium, killing sixty people and sickening thousands. After lengthy and careful investigation, Roholm determined that gaseous fluoride compounds were responsible. Roholm also identified aluminum smelters as emitters of large quantities of fluoride gases.
In the mid-1930s, whether natural or anthropogenic, fluoride compounds were nothing but bad news for human and environmental health.
Just as fluoride’s negative image was beginning to crystalize in the minds of scientists and public health officials, however, a countervailing set of ideas began forming. Ironically, it also stemmed from the work of Frederick McKay.

Dr. Trendley H. Dean in the 1950s (left). An 1885 advertisement for cocaine for dental pain in children (right).
As far as McKay could tell, the staining did not actually compromise the strength or physical health of teeth. On the contrary, people living in endemic brown-stain regions seemed to have fewer cavities than the general population.
The man who played the most important role in transforming fluoride’s medical image from tooth disfigurer to a potential prophylactic against dental caries—cavities that require either filling or removing teeth—was Trendley H. Dean. A St. Louis dentist who had joined the Army Dental Corps in World War I, Dean went on to become a key figure in public health dentistry. In 1930, he was appointed chief scientist of the newly established Dental Research Section of the Nation Institutes of Health, and then in 1948 became the first director of the National Institute of Dental Research.
A dentist and patient in the 1910s or 1920s.
Dean was quick to realize that solving the mystery of mottled enamel, though useful, was of secondary importance compared to the broader public health implications of dental caries. In a letter to the U.S. Surgeon General in 1932, Dean repeated McKay’s earlier observation that “individuals in an endemic [brown-stain] area show a lesser incidence of caries than individuals in some nearby non-endemic areas. Consequently, the study of mottled enamel may disclose some lead applicable to the vastly more important problem, dental caries.”
Once it became clear that fluoride was the cause of brown stain—which Dean would soon label dental fluorosis—Dean shifted the focus of his research, and that of the government’s health bureaucracy, from eliminating fluorosis to combatting caries.
Optimizing Nature
Dental caries was perceived as one of America’s most widespread health problems in the early twentieth century. Since dentists were comparatively few and dental surveys virtually nonexistent, it is difficult to know just how pervasive the condition was and to what extent, if at all, it had gotten worse over time.

Nonetheless, dentists themselves were convinced that it had reached epidemic proportions, a perception that appears to have been borne out by military fitness records. These show that in 1916, for example, one third of potential recruits failed their health exam due to caries-related problems. As a result, funds began to flow toward dental research, both from government sources and from corporate foundations.
Many dentists and medical scientists were convinced that Americans’ diets, particularly their fondness for refined flour and sugar, were largely to blame. But changing people’s dietary habits, then as now, seemed to be an insurmountable obstacle.
No wonder, then, that Dean and others were excited by the discovery of fluoride’s impact on teeth.
During the 1930s, Dean, McKay, and colleagues from the PHS and various university dental schools set about trying to demonstrate fluoride’s connection to both dental fluorosis and reduced rates of caries. Although nobody understood exactly how it worked—and nobody would for a long time—fluoride did indeed seem to change the structure of teeth in a way that offered some protection against the assaults of the 20th century American diet.
Embarking on a succession of epidemiological studies in towns that had fluoride-rich water supplies, Dean was able to gradually zero in on a ratio that appeared to offer considerable protection against caries while causing limited and barely discernable fluorosis. The magic number, he determined, was 1 part per million (1ppm).
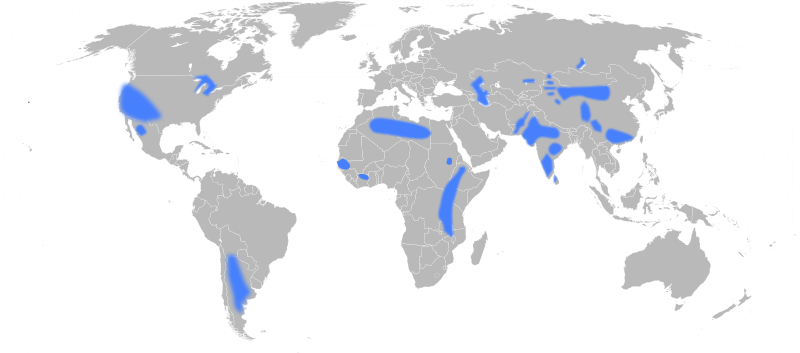
A 2009 map depicting areas with groundwater fluoride concentrations above the recommended levels.
As the studies continued, Dean and his colleagues published a series of articles that would become the scientific bedrock of fluoridation. So although water naturally containing 1ppm fluoride existed in very few places, it nonetheless came to be seen as the optimal level, and water containing less was deemed “fluoride deficient.”
Dean himself did not advocate artificially augmenting the level of fluoride in drinking water, at least not during the 1930s and 1940s. A cautious and methodical researcher, he felt that many years of further investigation would be required before such a prospect could be contemplated. Even the American Dental Association, subsequently fluoridation’s most steadfast advocate, was reluctant to endorse the idea. However, some dental researchers were less circumspect.

In the early 1940s, Dean began to explore the possibility of testing artificial fluoridation in a handful of carefully chosen communities. After consulting with colleagues at the University of Michigan, Dean selected the towns of Grand Rapids and Muskegon to participate in a 15-year fluoridation trial. Both cities drew their water, which had virtually no natural fluoride, from Lake Michigan. In January 1945, with the enthusiastic cooperation of city officials, Grand Rapids began adding sodium fluoride—a waste product of aluminum production—to its water supply while Muskegon remained fluoride free.
But not everyone was prepared to wait fifteen years.

In the mid-1940s, a small group of activist dentists in Wisconsin began agitating for immediate water fluoridation. Chief among them was John Frisch, a Madison dentist and prominent member of the Wisconsin State Dental Society. Frisch had been following McKay and Dean’s work closely throughout the 1930s.
In his mind, Dean’s publications had firmly established that water containing around 1ppm fluoride was both efficacious and completely safe in the fight against dental caries. Artificial fluoride, he was convinced, was no different from natural fluoride, and endless trials and experiments would merely condemn another generation of children to the pain and misery of caries. So sure was Frisch of fluoride’s safety and efficacy, that he began to add it to his home water supply so that he could monitor any changes in his children’s’ dental health.
While the Grand Rapids trial was in its infancy, Frisch and his colleagues began to promote fluoridation throughout Wisconsin. In 1947, after two years of fierce campaigning and politicking, they finally convinced city officials to fluoridate Madison’s water supply.
To allay skeptical members of the public, for whom fluoride was primarily an ingredient in rat poison, Frisch began to increasingly invoke fluoride’s “naturalness.” “The dental profession,” he declared in 1946, “is imitating nature as closely as it can.” One only had to look to Green Bay, “where people have been drinking fluorine water with sodium fluoride supplied to it by nature for over one hundred years, and no deleterious effect of any nature has occurred in that locality.”

The logo for the American Dental Association.
While the Michigan trials continued, with dozens of dental researchers descending on the state to prod and probe children’s teeth for signs of caries, Frisch and his acolytes barnstormed Wisconsin, lobbying community after community to add small amounts of sodium fluoride to its water supply.
Without any long-term toxicological studies—and apparently without any qualms—water authorities complied. Frisch’s self-proclaimed goal was to have 50 communities fluoridated by 1950, a target he duly reached.
While these numbers were quite remarkable given the rising grassroots opposition to fluoridation, Frisch would not be satisfied until every citizen in America, or for that matter, the world, could enjoy the benefits of fluoride compounds in their drinking water. Thus he grew alarmed when technical problems with the fluoride injection equipment, along with concerns about adequate sodium fluoride supplies, threatened to slow the pace of adoption.

Those concerns prompted him to write to ALCOA, which produced large quantities of sodium fluoride waste as part of its production process. “The demand for this material,” he informed them, “will soon reach astronomical proportions.” If Frisch had any qualms about the “naturalness” of ALCOA’s sodium fluoride, he managed to suppress them. But it seems unlikely that he did.
By 1950, the trial in Grand Rapids was yielding very positive results. In fact, officials in Muskegon grew increasingly agitated. From their perspective, the town’s ongoing status as the control city meant their citizens’ dental health was being sacrificed on the altar of Dean’s scientific cautiousness. They would indeed begin fluoridation in 1951, thus severely compromising Dean’s 15-year study in the eyes of fluoridation skeptics.
Meanwhile, the Wisconsin fluoridationists’ most important victory occurred at the federal level.
Frisch and his colleague, Frank Bull, the State Dental Director of Wisconsin, began to lobby the PHS to endorse water fluoridation. With anti-fluoridationist influence growing—they had already defeated several fluoridation drives in Wisconsin—Frisch and Bull were concerned that by the time Dean’s Grand Rapids trial was completed, fluoridation might be politically dead.

The PHS caved in to the pressure remarkably quickly, endorsing fluoridation in June 1950 and strongly supporting the practice thereafter. The move had a cascading effect. Within months, the American Dental Association, the American Water Works Association, the American Medical Association, and a host of other high-profile government bureaus and professional bodies all gave fluoridation their stamp of approval.
For Frisch, the PHS endorsement represented a monumental victory for dental public health. For others, it also represented new economic opportunities.
In 1951, for example, the trade journal Chemical Week enthusiastically proclaimed the coming “Water Boom for Fluorides.” “The market potential,” the author gushed, “has fluoride chemical makers goggle-eyed.” In sum, “it all adds up to a nice piece of business on all sides, and many firms are cheering the USPHS and similar groups on as they plump for the increasing adoption of fluoridation.”
Fluoridation skeptics seized upon such articles as evidence of the impure motives of fluoridation advocates. ALCOA’s involvement in discovering fluoride in water—and supplying sodium fluoride to water authorities—only deepened their skepticism.
The whole fluoridation story, in fact, lent itself remarkably well to conspiracy theorizing. For example: Andrew W. Mellon, a founder of ALCOA and one of its major stockholders, was the U.S. Treasury Secretary from 1921-1932, when the PHS was still a division of the Treasury Department. It was therefore Mellon’s PHS that ordered Dean to study fluoride in the first place. Fluoride’s transmogrification from toxic waste to public health miracle, skeptics argued, suited American industry all too well.
The PHS endorsement was certainly a major public relations victory for fluoridationists. It also meant that ALCOA and other suppliers of fluoride compounds could in good conscience take advantage of a new business opportunity.
However, it by no means guaranteed the spread of fluoridation. Neither the PHS nor any other federal or state body had the power to mandate nationwide fluoridation. Instead, the decision was left up to cities and towns throughout the country. And fluoridation skeptics had more influence over local referenda than over federal or state government agencies.

Scientists and dental researchers such as Dean were flabbergasted and appalled by the array of charges hurled at them from an assortment of activists that ran the gamut from skeptical doctors and dentists to unhinged anti-communist zealots, the latter famously parodied by Stanley Kubrick in his classic 1964 film, “Dr. Strangelove,” in which a character frets over the contamination of Americans’ “precious bodily fluids.”
Continued anti-fluoridationist lobbying culminated in 1954 with the introduction of a congressional bill that threatened to outlaw fluoridation altogether. Submitted by Roy Wier, a Democrat from Minnesota, H.R. 2341 was designed “to protect the public health from the dangers of fluorination of water.” The stakes could not have been higher: had the bill passed, it would have made it illegal for any government agency at any level to introduce fluoride into its water supply.
With over two decades of crucial involvement in fluoride and dental health issues and a list of 46 scientific papers on the subject, Dean was fluoridation’s star witness at the H.R. 2341 hearings. Allaying fears that fluoridation constituted mass medication without the consent of the targeted population, Dean insisted that fluoridation was neither a treatment nor a cure for caries. Rather, “Fluorine simply prevents the decay from developing.”

“In short,” he declared in an appeal to the moral authority of nature, “fluoridation of public water supplies simulates a purely natural phenomenon—a prophylaxis which Nature has clearly outlined in those communities that are fortunate enough to have about one part per million of fluoride naturally present in the public water supply.”
Wier’s bill languished as did anti-fluoridationists’ best chance of ending the practice. Subsequently, Dean’s naturalization of water fluoridation became the standard language of government agencies, the American Dental Association, and countless water authorities throughout the U.S. and other nations that adopted it.
Far from constituting a form of alchemical sleight of hand by which industrial pollution was converted into forced mass medication, as opponents charged, proponents of fluoridation argued that adding fluoride to drinking water was merely a case of optimizing nature: a slight tweak to adjust a chemical benefit that “Nature has clearly outlined.”

Fluoride Ecology
The fluoride consensus developed a dozen years before the publication of Silent Spring and the rise of the modern environmental movement. By the time environmentalism started to gain political traction in the 1960s and 1970s, fluoridation enjoyed enormous support among the scientific and policy elite.
By the late 20th century, fluoride was commonly added to most toothpaste and various other dental products, while numerous foods were fumigated with fluoride chemicals. Fluoride compounds also appeared in fast food wrappers.
Despite the mid-20th century scientific consensus, fluoride skepticism did not disappear. Scientists, dentists, and medical doctors who opposed the practice continued to publish books and articles insisting that fluoridation was problematic. Paul Ehrlich expressed this concern in 1977: “The scientific evidence supporting the efficacy and safety of mass fluoridation at the generally recommended level … is not as good as it ought to be.”
Some skeptics claimed it caused various cancers or Down Syndrome. Others contended that it was likely responsible for a raft of low-level chronic conditions. For example, people suffering from mild forms of skeletal fluorosis exhibited the same symptoms as those afflicted with arthritis, and most physicians were not trained to distinguish between the two.
In addition, several high-profile experts who had previously supported fluoridation changed sides and began to oppose the practice.
By the early 21st century, even some fluoridationists became concerned that people were being exposed to too much fluoride, a fact reflected in the 2011 EPA recommendation that water authorities reduce fluoride levels from 1ppm to 0.7ppm.

Iodized salt with fluoride sold in Germany (left). Colgate, like most American toothpastes, indicates the presence of fluoride by including it as part of the name (right).
FSA, which became the most commonly used water fluoridation product from the 1970s onward, also came under renewed scrutiny.
William Hirzy, a former senior scientist at the EPA, argued that the arsenic present in FSA likely contributed annually to hundreds of cases of lung and bladder cancer. Hirzy insisted that only pharmaceutical grade sodium fluoride should be added to drinking water, a change that would prohibitively increase the cost of fluoridation.
Meanwhile claims for fluoridation’s efficacy in reducing cavities have been revised downward significantly: in the 1950s, dentists claimed it reduced caries by over 60%, while today they offer a more modest 25%. Meta studies over the past two decades suggest both figures are inflated. Moreover, rates of dental fluorosis have increased markedly in the past quarter of a century. Despite all the charges and mounting evidence, pro-fluoridationists continued to insist that fluoridation remained a safe and necessary public health measure.

A sticker on a city electrical box in Vancouver, WA from Info Wars, a right-wing media organization known for promoting conspiracy theories (left). Signs from a 2009 protest against fluoride in Australia (right).
Non-fluoridating nations such as Sweden and France have shown that it is possible to reduce dental caries without having to engage in a practice with which a substantial proportion of the population has always felt uneasy.
No doubt those countries owe a debt to people such as McKay and Dean for demonstrating a link between fluoride and dental caries. However, it is now clear that the benefits of fluoride are primarily topical. Thus fluoridated toothpaste, rather than drinking water, has in all likelihood been the greatest contributor to fighting cavities, along with improvements in diet and overall dental health.
In fact, communities that have stopped fluoridation have not experienced an increase in dental caries. Furthermore, dental health in regions which have never fluoridated their water is not significantly different from fluoridated regions. In Canada, for example, non-fluoridated British Columbians actually have fewer cavities than fluoridated Ontarians.
One result of the long-term argument is that the fluoridationists, who are understandably frustrated by the worst excesses of the anti-fluoridationists, treat fluoridation like a sacred cause to be defended at all cost. As a result, they cling to an ahistorical view that ignores the context in which fluoridation was initially promoted and the ecological and scientific changes that have occurred since.

A 2009 protest in San Francisco, CA against fluorinated water.
One can accept that fluoridation was a defendable public health measure in the mid-20th century. Tooth decay was a serious problem, and it was arguably worth taking some risks in order to tackle it. However, the continued insistence among public health authorities and dentists that community water fluoridation remains essential to good dental health is incommensurate with evidence for its effectiveness, as well as downplaying the harm of fluorosis and other problems.
In all likelihood, the only significant problem that would arise from an end to fluoridation is that the Florida phosphate industry would have to find a different way—no doubt one more expensive and less convenient—to dispose of its toxic waste.
Read more from Origins on public health: Vaccination; Influenza Pandemics; The Guatemala Inoculation Experiments; Flu History; Vaccines and Parenthood; The 1918 Pandemic.
Bryson, Christopher. The Fluoride Deception (New York: Seven Stories Press, 2004).
Carstairs, Catherine. “Debating Water Fluoridation Before Dr. Strangelove,” American Journal of Public Health, Vol 105, No. 8 (August 2015): 1559-1569.
Carstairs, Catherine. “The Environmental Critique of Water Fluoridation,” Scientia Canadensis, 38:1 (2015): 1-21.
Connett, Paul, James Beck, and H. S. Micklem, The Case against Fluoride: How Hazardous Waste Ended Up in Our Drinking Water and the Bad Science and Powerful Politics That Keep It There (White River Junction, Vermont: Chelsea Green Publishing, 2010).
Drake, Brian. Loving Nature, Fearing the State: Environmentalism and Antigovernment Politics before Reagan (Seattle: University of Washington Press, 2013).
Freeze. R. Allan and Jay H. Lehr. The Fluoride Wars: How a Modest Public Health Measure Became America’s Longest-Running Political Melodrama (Hoboken, NJ: John Wiley & Sons, 2009).
McNeil, Donald R. The Fight for Fluoridation (New York: Oxford University Press, 1957).
Melosi, Martin. Precious Commodity: Providing Water for America’s Cities (Pittsburgh: University of Pittsburgh Press, 2011).
Picard, Alyssa. Making the American Mouth: Dentists and Public Health in the Twentieth Century (New Brunswick, NJ: Rutgers University Press, 2009).
Sellers, Christopher. “The Artificial Nature of Fluoridated Water: Between Nations, Knowledge, and Material Flows,” Osiris 19 (2004): 182-200.

