As of 2020, the CDC estimates that just over one in ten Americans has diabetes, a chronic condition that can have serious, life-threatening complications if left untreated.
Diabetes is caused when the body fails to produce insulin (Type I diabetes) or does not properly use it (Type II). Insulin is a hormone made by the pancreas that manages metabolism and directs the body to store glucose in fat, muscles, and the liver. Without it, glucose levels in the bloodstream can rise to dangerously high levels.
Although observations and attempted treatments of diabetes date back to ancient times, the most important milestone occurred when a new treatment—insulin injection—was first successfully used on January 23, 1922.
The efforts to refine and distribute insulin therapy represented an important endpoint in the development of medical treatment. It also raised lasting bioethical questions over how to ensure the widescale accessibility of life-saving drugs while maintaining their affordability.
The earliest references to the disease date to the ancient Egyptians, over a millennium before the common era. Later, in the second century CE, the Greek physician Aretaeus of Cappadocia invented the term “diabetes” itself, which derived from the word for “siphon”—to pass through—and which described the increased urination often associated with the condition.

Early descriptions of the disease were crude, emphasizing abnormalities in the urine like excessive sweetness (even if the excess sugar that caused it was not discovered until 1776 by the Liverpool physician Matthew Dobson).
Treatments were similarly rudimentary, involving concoctions of herbs and seeds and, by the 18th century, dietary changes. In 1870, French pharmacist Apollinaire Bouchardat most famously observed the importance of a sugar-free diet during the Franco-Prussian War.
Research into the function of the pancreas, although a medical tradition dating back to the 4th century BCE under the ancient Greeks, assumed a more modern form by 1869 when German medical student Paul Langerhans first described “islets” (floating clumps of cells) scattered about the organ. These cells released enzymes distinct from the other digestive enzymes produced by the pancreas and, although Langerhans could not yet describe their function, his discovery set the stage for further research.
After the First World War, a new generation of medical researchers took up the study of the pancreas. Inspired by Langerhans’s work, Canadian orthopedic surgeon Frederick Banting became fascinated by the relationship between the pancreas and diabetes, as well as the challenges of isolating the excretions of the islets from other pancreatic tissue.

Banting knew that the laboratory of physiologist J.J.R. Macleod, an internationally recognized expert on carbohydrate metabolism at the University of Toronto, was studying the pathology of diabetes. Even though he was inexperienced with laboratory research, beginning in the late spring of 1921, Banting joined Macleod’s laboratory, where he was given one undergraduate assistant, Charles Best (selected by coin toss), to study and isolate the islet’s excretion.
Banting and Best’s experiments proved successful. They collected a sample of liquid insulin in a pure state on July 30, 1921 (which they initially called it “Isletin”), isolated from the islets of Langerhans in the pancreases of dogs. After testing their extract in a series of diabetic dogs, Banting and Best observed rapid reductions in blood sugar in the animals.

When they first reported their results to Macleod that fall, he was unconvinced. He insisted on further testing, which soon confirmed Banting and Best’s early results. In December, Macleod recruited a fourth scientist, James Collip, a clinical biochemist, to help refine the process of producing the pure insulin extract. Collip also demonstrated that bovine insulin, collected from pancreases of slaughtered cattle rather than laboratory dogs, was equally efficacious and was more easily procured.
The first human trial of insulin therapy began on January 11, 1922. Fourteen-year-old Leonard Thompson was given an injection of insulin at the Toronto General Hospital. The experiment failed. The 15 ml dose of insulin was insufficient to treat his deadly ketoacidosis, and Thompson suffered an allergic reaction.
Over the following 12 days, Collip refined the concentration of the dosage before another test was performed on Thompson on January 23, this time with success. Thompson’s blood sugar and glycosuria dropped and his ketonuria disappeared.
Within two months, Banting and his team published their results. In April, Macleod suggested renaming the refined extract “insulin” (from the Latin for “island”) to distinguish it from Banting and Best’s original crude extraction “Isletin.”
Elizabeth Hughes, daughter of U.S. Secretary of State Charles Hughes, became an example of success among the early patients. She arrived in Toronto on August 15, 1922 to receive insulin therapy beginning the following day. At the time, she was 14 years old, 5 feet tall, weighed 45 pounds, and was so weak she could barely walk, according to Banting’s notes.
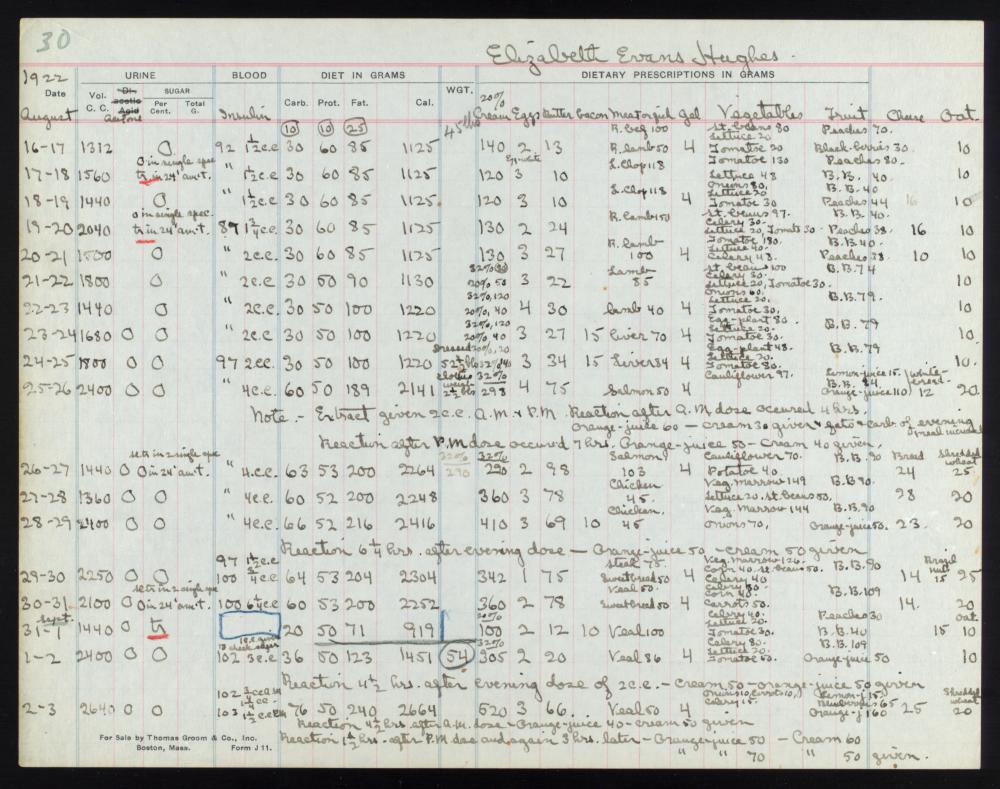
When she returned home roughly three months later, she was healthier, more than twice her original weight, and had become a shining example of the power of insulin therapy for diabetic children. The following year, Macleod and Banting were awarded the Nobel Prize in Medicine for their pathbreaking work.
Their discovery made, its efficacy proven, the question nevertheless remained of how to patent it. Macleod and Banting feared a patent for a life-saving medical tool was ethically unsound, especially if its manufacture was monopolized by a private company.
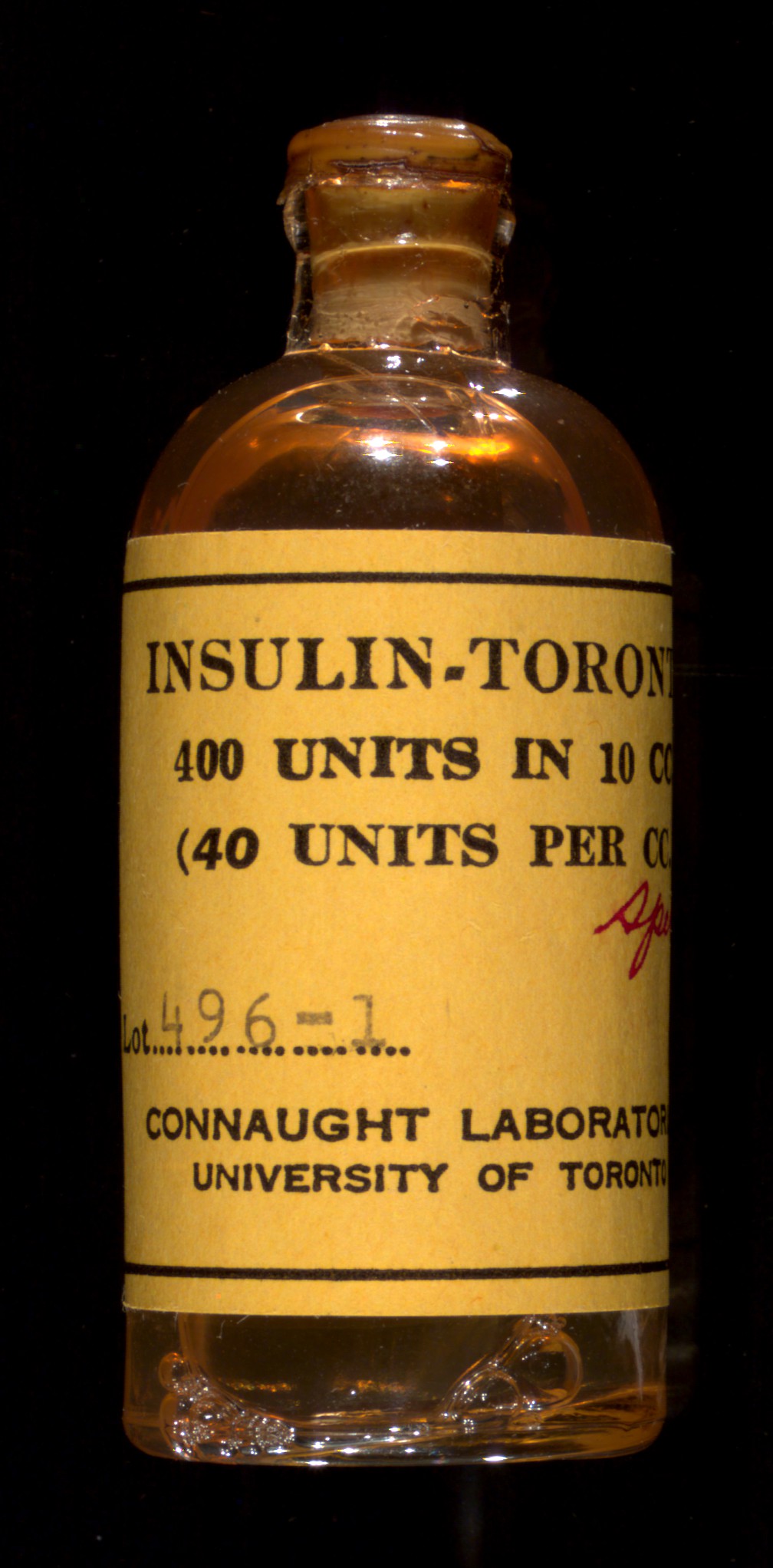
But their discovery—along with Collip’s procedure for culturing insulin from bovine pancreases in a laboratory, for which he did wish to pursue a patent—needed to be scaled up to manufacture life-long treatments for diabetic children. Furthermore, the insulin they collected and tested only lasted for a short time: only in partnership with scientists at a large pharmaceutical partner would the team eventually be able to manufacture insulin at scale.
After much debate, and to ensure quality control, the four original collaborators, Banting, Best, Collip, and Macleod, agreed to sell the patent for their discovery to Connaught Laboratories at the University of Toronto for a token payment of one dollar. Meanwhile Eli Lilly was given a one-year exclusive license to produce insulin commercially.
Industry research has continued to build upon the work of the four co-discoverers. In the 1970s, research began on synthetic insulin (to mitigate allergies to animal products). Eli Lilly brought fully synthetic insulin to market in 1982 as Humilin. Subsequently, the company began research on genetically modified human insulin, which has been sold as Humalog since 1996.
While each new formulation provided more reliable control of diabetes, it also renewed patents on a life-saving drug. This has resulted in soaring costs that stand in stark contrast to Banting’s vision of a readily accessible drug. The cost of one vial of Humalog, for example, rose from $21 in 1999 to $332 in 2019.
Bringing these costs down, and restoring Banting’s dream that live-saving insulin should be as widely accessible for as cheap as possible, is dilemma that bioethicists are still seeking to resolve.
